Abstract
Background: The number of hematopoietic cell transplantation (HCT) in older patients has been increasing. Although donor selection is a key factor for better outcomes, older patients frequently lack HLA-matched sibling donors (MSD) due to their comorbidities. Previous studies have shown that increased donor age is associated with a high mortality. In this study, we sought to identify the best donors for older patients and evaluated appropriate alternatives for MSD.
Patients and methods: For this retrospective cohort study, clinical data of donors and recipients were obtained from the registry data of the Japanese Society for Transplantation and Cellular Therapy (JSTCT). This study included 9,687 patients between the ages of 50-75 with acute leukemia or myelodysplastic syndrome who received the first HCT between 2011 and 2020 from MSD (N=1,317), 8/8 HLA-matched (HLA-A, -B, -C, and -DRB1 allele-level) unrelated donors (8/8 MUD) (N=2,341), 7/8 HLA-matched unrelated donors (7/8 MUD) (N=1,371), haploidentical related donors (HRD) (N=721), or single-unit cord blood (CB) (N=3,937). Only donors over 40 years of age were chosen to become MSD. For HRD, at least two-antigen (HLA-A, -B, -DR antigen-level) mismatched donors were included, and for CB donors, a maximum of two-antigen mismatched donors were included. Risk factors for overall mortality and other endpoints were analyzed using Cox proportional hazards models and Fine and Gray's proportional hazards models, respectively.
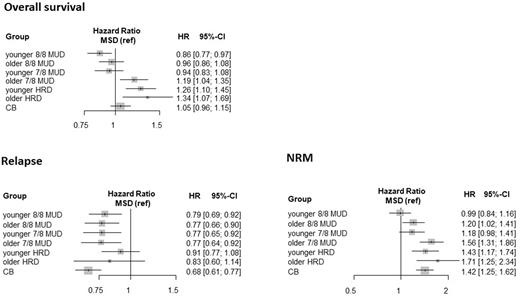
Results: The median donor age excluding CB was 42 years (range, 13-73). The unadjusted probability of overall survival (OS) at five years post-transplantation was highest in HCT from younger (<40 years) 8/8 MUD (48% [95% CI, 44.7%-51.2%)] followed by those from older 8/8 MUD (46% [95% CI, 42.7%-49.6%)]. The probability of OS in HCT from MSD was 43% (95% CI, 39.9%-46.0%). After adjusting for other significant factors in multivariable analysis, younger 8/8 MUD were significantly associated with a higher rate of survival (adjusted hazard ratio [aHR], 0.86; 95% CI, 0.77-0.97, P=0.012), compared to MSD (Figure), especially in patients with high-risk disease (aHR, 0.83; 95% CI, 0.71-0.97, P=0.018). On categorization of recipient age by 5 years, survival in younger 8/8 MUD was higher than that in MSD, and that trend became more apparent as recipient age increased (P for trend=0.002). On the other hand, survival in MSD was comparable to that in older 8/8 MUD, younger 7/8 MUD, and CB. Relapse rate in MSD was significantly higher than those in other donors except for HRD, while non-relapse mortality (NRM) in MSD was significantly lower except younger 8/8 or 7/8 MUD (Figure). Incidence of grades III-IV acute graft-versus-host disease (aGVHD) was significantly lower in younger 8/8 (aHR, 0.66; 95% CI, 0.50-0.86, P=0.002) or 7/8 MUD (aHR, 0.72; 95% CI, 0.52-0.98, P=0.039) than MSD. As for a graft source, no significant difference between bone marrow and peripheral blood stem cells was observed in survival.
Conclusions: Younger 8/8 MUD is the first choice as a donor source in older patients due to both low rates of relapse and NRM. The second choice is MSD, older 8/8 MUD, younger 7/8 MUD, and CB. This study will provide older patients with more opportunities to find appropriate donors in suitable timing. Reducing NRM may be a key for HLA-mismatched donors to be an alternative donor option.
Figure: Forest plots of donor groups for overall survival, relapse, and non-relapse mortality (NRM). MSD, HLA-matched sibling donors; MUD, HLA-matched unrelated donors; HRD, haploidentical related donors; CB, cord blood
Disclosures
Kanda:CSL Behring K.K.: Honoraria; MSD K.K.: Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria; AbbVie Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Ono Pharma Inc.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin Co., Ltd.: Honoraria; Sanofi K.K.: Honoraria; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; TEIJIN PHARMA LIMITED.: Honoraria; Novartis Pharma K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb Co: Honoraria; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; ASAHI KASEI PHARMA CORPORATION: Honoraria; asclepia: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; NIPPON KAYAKU CO.,LTD.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Eisai: Research Funding. Sawa:Astellas Pharma Inc.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Ono Pharmaceutical Co., Ltd.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Pfizer Japan Inc.: Honoraria; MSD K.K.: Honoraria; Bristol-Myers Squibb K.K.: Honoraria; Asahi Kasei Pharma Corp.: Honoraria; Novartis Pharma K.K.: Honoraria; Eisai Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Sanofi K.K.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; Celgene K.K.: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Shire plc: Honoraria; Mundipharma K.K.: Honoraria; AbbVie G.K.: Honoraria; CSL Behring K.K.: Honoraria; SymBio Pharmaceuticals Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; AstraZeneca K.K.: Honoraria; Daiichi Sankyo Co., Ltd.: Honoraria; GSK plc.: Honoraria. Atsuta:Kyowa Kirin Co., Ltd: Honoraria; Astellas Pharma Inc.: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma KK: Honoraria; AbbVie GK: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal